KLH: Keyhole Limpet Hemocyanin
KLH: Keyhole Limpet Hemocyanin – biosyn native KLH
The hemocyanin produced from the hemolymph sera of the marine mollusk Giant Keyhole Limpet, Megathura crenulata, has been in use and marketed as a crude or partially purified product for over 40 years by some chemical companies. This molecule is well recognized and commonly abbreviated as KLH. The crude research grade KLH is used in antibody production in animals against antigens. The animal sera containing the antibody for the antigen are further processed and used as immunological reagents or in immunological assays. The partially purified product has also been used in early human trials for immune status evaluation.
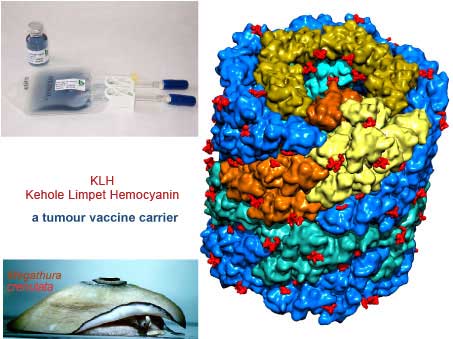
KLH structure
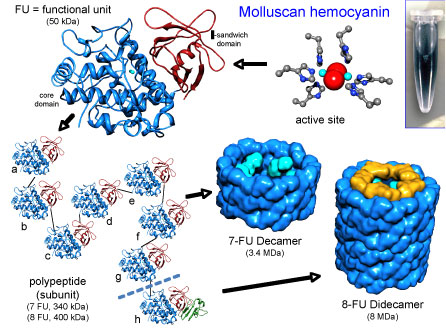
The native KLH is a cylindrical copper containing blue protein with a molecular mass ranging from 8-32 MDa (Million Dalton). Electron microscopy structural studies reveal presence of two oligomeric forms:
- Didecamers with a molecular mass of 8 MDa, are present in the form of hollow cylinders. They are approximately 35 nm in diameter and 40 nm in length.
- Multidecamers with a molecular mass of 12-32 MDa, present as stacked decamers (multidecamers) of varying length, added on one or both sides of a “nucleating” didecamer. Single decamers exhibit rectangular side views.
The didecamer consists of 20 subunits and occurs in two different Isoforms named KLH1 and KLH2. The subunits are biochemically and immunologically distinct. Analysis of KLH by native-PAGE gives two characteristic bands, one corresponding to KLH1 with an apparent molecular mass of about 390 kDa (kilo Dalton) and the other corresponding to KLH2 with an apparent molecular mass of 350 kDa.
Each subunit of both Isoforms, KLH1 and KLH2, consists of eight paralogous functional units (FU), named ‘a‘ to ‘h‘, whereby the FUs a-f form the wall of the cylinder and g-h build up the collar-complex of the molecule. The molecular mass of the functional units is about 50 kDa. Based on this information it is expected that the molecular masses of the KLH1 and KLH2 subunits are approximately 400 kDa. The functional unit (FU) within the subunit contains a binuclear copper binding site that reversibly binds molecular oxygen.
The copper is in the cuprous [Cu(I)] state, and oxygen-binding generates light absorbance in the near ultraviolet around 343 nm and imparts the characteristic blue color to the molecule. The deoxygenated molecule is colorless.
Peptide sequence
The peptide sequence surrounding the two copper-binding sites is highly conserved, with three copper-liganding histidines in both cases. The active site geometry and molecular architecture of the mollusk hemocyanin differ from those of arthropod hemocyanin.
Removal of second copper from mollusk hemocyanin by cyanide ions is both slower and more difficult compared to arthropod hemocyanin, where all the copper comes off readily. Further differences between the two hemocyanin subunits are revealed by hydrogen peroxide treatments.
