Selenase® Reduces Mortality
Initial Serum Selenium Levels in Sepsis Compared to Reference Values
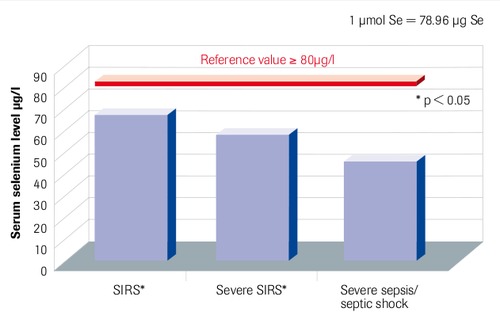
“Patients with SIRS and sepsis have low selenium levels” Sakr et al. 2007
Maximal serum selnium levels in intensive care patients compared to APACHE II and SAPS score
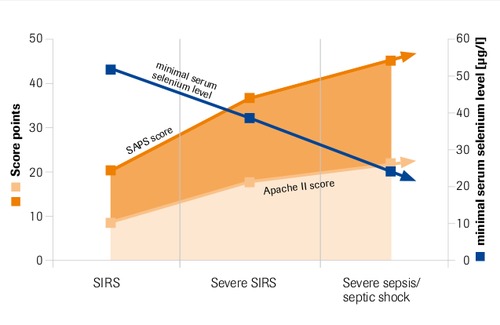
“Selenium levels correlate inversely with severity of disease and risk of mortality” Sakr et al. 2007
Minimal serum selenium levels in Intensive Care Patients Correlate with Outcome
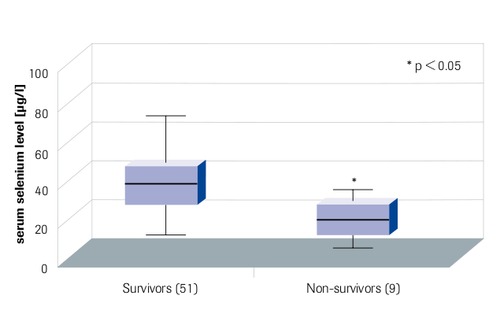
“Survivors have higher selenium levels” Sakr et al. 2007
Change of Mortality During Selenase® Supplementation
“selenase® administration improves prognosis”
Significant reduction of:
- inflammatory reaction (Zimmermann et al. 1997)
- free radical burden (Zimmermann et al. 1997)
- acute renal failure (Angstwurm et al. 1999)

Selenium in Intensive Care (SIC)
Prospective, randomised, double-blind, Phase III multi-centre study in patients with SIRS/Sepsis
28-day mortality Intention-to-treat analysis Angstwurm et al. 2007
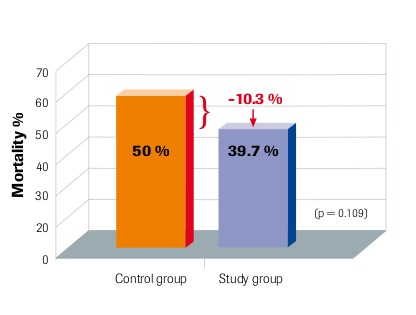
“Selenium levels correlate inversely with severity of disease and risk of mortality” Sakr et al. 2007
28-day mortality Per-protocol group Angstwurm et al. 2007
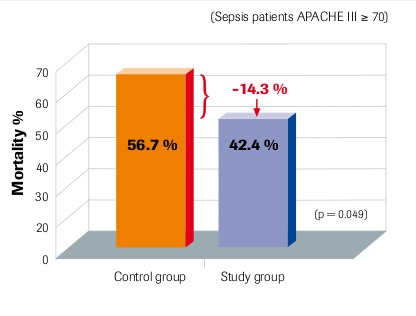
“Selenium levels correlate inversely with severity of disease and risk of mortality” Sakr et al. 2007
“selenase® significantly reduces mortality”
SIC-Study Subgroups
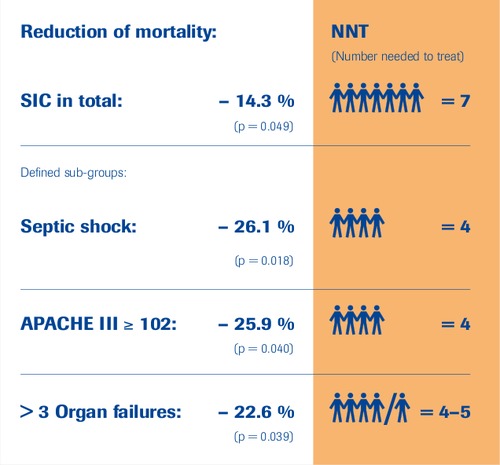
“selenase® is efficacious”
Duration of survival according to Kaplan-Meier
Intention-to-treat analysis Angstwurm et al. 2007
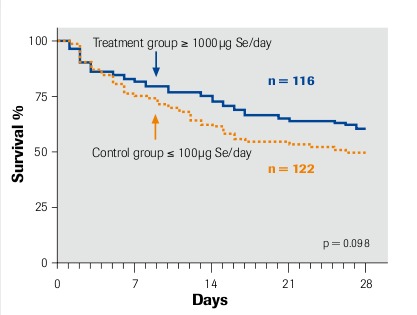
Per-protocol group Angstwurm et al. 2007

“selenase® significantly prolongs survival” μmol Se = 78.96 μg Se
Duration of survival according to Kaplan-Meier
Selenium consumption is particularly high in the acute phase of sepsis/SIRS
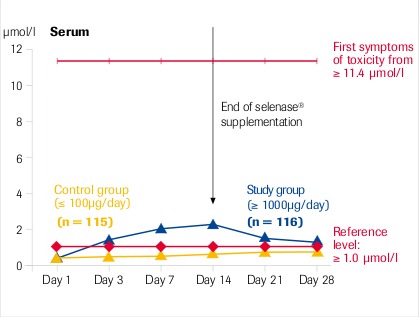
Intracellular selenium concentration (whole blood) is the decisive factor for its action
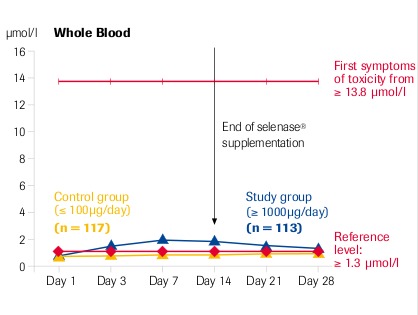
“High dose selenase® supplementation is safe” μmol Se = 78.96 μg Se
Selenase® Affects Central Metabolic
Pathophysiology of SIRS/Sepsis
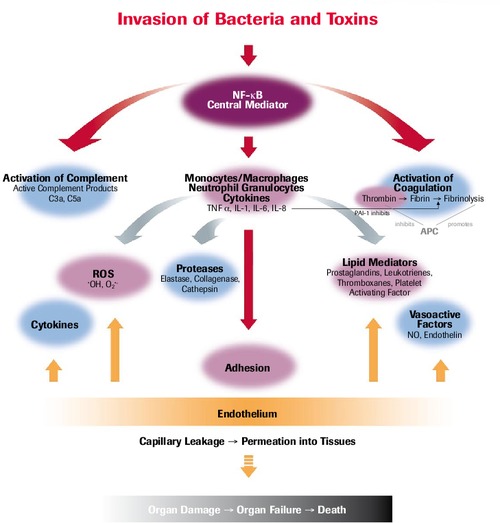
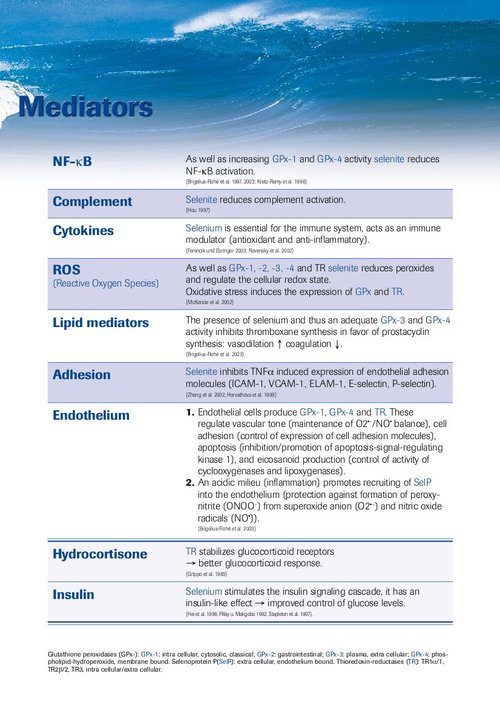
Meta-analysis: Selenium in Intensive Care Patients
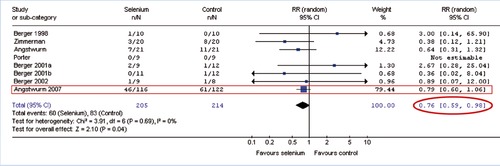
Heyland et al. 2007∗ (exkl. Kuklinski 1991) ∗ Daren K Heyland, Kingston, Canada: ISICEM 2007, www.criticalcarenutrition.com “The benefit from selenium is evident”
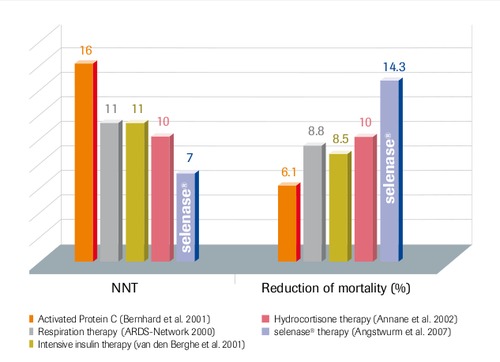
Comparison of Therapeutic Options
“selenase®: progress in sepsis therapy”
Guidelines for Selenium
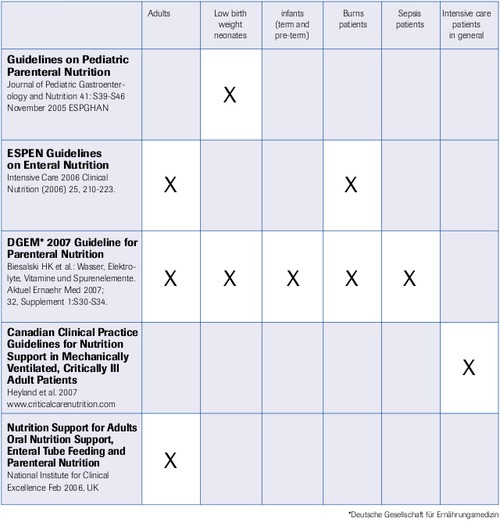
“Selenium is a must” (M. Berger, Lausanne 2007, ISICEM)
Selenium in Burns Patients
- 41 patients with thermal burns 0.8 (BSA > 20 %)
- Supplementation in the study group 8-21 days
- Study 1: 315 μg Se/d, 2.5 mg Cu/d, 26.2 mg Zn/d
- Study 2: 380 μg Se/d, 3.1 mg Cu/d, 31.4 mg Zn/d
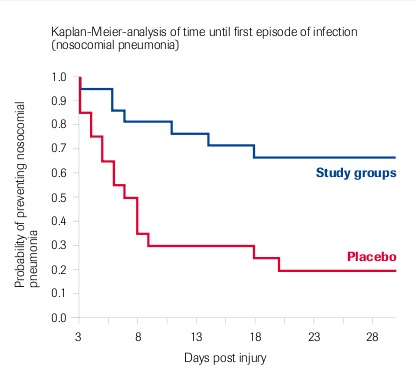
Berger et al. 2006: Meta-analysis of 2 studies (1993-1996 and 1998-2003) – Randomised, double-blind, placebo-controlled – “Lower incidence of nosocomial pneumonia”
μmol Se = 78.96 μg Se
Selenium in Burns Patients
Individual Results: Significant Reduction Of
- Number of nosocomial pneumonias
- Number of infectious episodes
- Duration of antibiotic therapy
- Duration of ICU stay
Berger et al. 2006: meta-analysis of 2 studies (1993-1996 and 1998-2003) Randomised, double-blind, placebo-controlled “Selenium reduces costs”
Tolerability of selenium
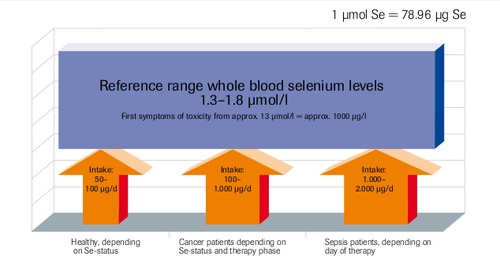
Literature at biosyn
Information for Healthy Individuals
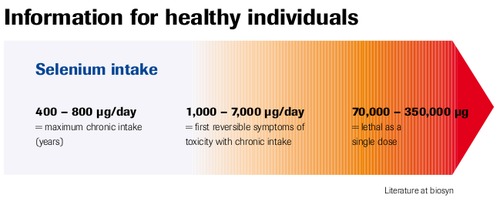
Literature at biosyn
Selenium Metabolism (Simplified)
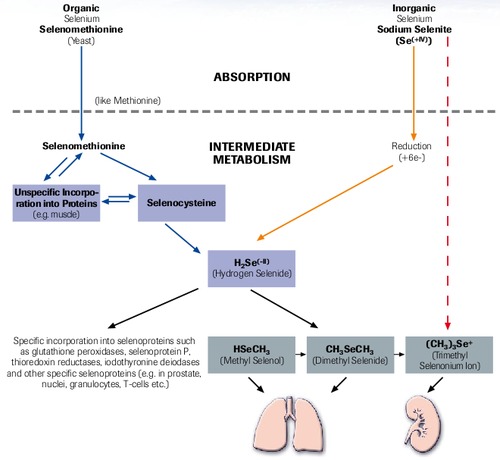
“selenase® has optimal bioavailability”
Dosage Recommendation∗
SIRS/Sepsis

Literature at biosyn
Multiple Trauma, Cranial Trauma, Burns, Acute Pancreatitis, Acute Myocardial Infarction

Literature at biosyn
Dosage Recommendation∗
Total Parenteral Nutrition

Literature at biosyn Recommendation for the administration of selenase®:
- separately from other infusions, if the pH is lower than 7
- at least 1 hour apart from administration of vitamin C
Reference Values
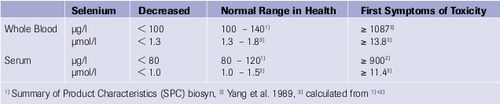
∗Further information on dosage and application see national SPC.
Selenase® Corrects Selenium Deficiency

Literature:
- Angstwurm MWA, Engelmann L, Zimmermann T, Lehmann C, Spes CH, Abel P, Strauß R, Meier-Hellmann A, Insel R, Radke J, Schüttler J, Gärtner R: Selenium in intensive care (SIC) study: Results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med 35 (2007) 1-9.
- Angstwurm MW, Engelmann L, Zimmermann T, Lehmann C, Spes CH, Abel P, Strauss R, Meier-Hellmann A, Insel R, Radke J, Schuttler J, Gartner R: Selenium in Intensive Care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med. 2007 Jan;35(1):118-26.
- Angstwurm MWA, Schottdorf J, Schopohl J, Gaertner R: Selenium replacement in patients with severe systemic inflammatory response syndrome improves clinical outcome. Critical Care Medicine 27 (1999) 1807-1813.
- Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaut P, Bellissant E. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002 Aug 21;288(7):862-71.
- ARDS-Network 2000: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000 May 4;342(18):1301-8.
- Berger MM, Eggimann P, Heyland DK, Chiolero RL, Revelly JP, Day A, Raffoul W, Shenkin A: Reduction of nosocomial pneumonia after major burns by trace element supplementation: aggregation of two randomised trials. Crit Care. 2006;10(6):R153.
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr; Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001 Mar 8;344(10):699-709.
- Brigelius-Flohé R, Banning A, Schnurr K. Selenium-dependent enzymes in endothelial cell function. Antioxid Redox Signal. 2003 Apr;5(2):205-15.
- Brigelius-Flohé R, Friedrichs B, Maurer S, Streicher R: Determinats of PHGPx espression in a cultured endothelial cell line. Biomedical and Environmental Sciences 10 (1997) 163-176.
- Clark LC, Combs GF Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL Jr, Park HK, Sanders BB Jr, Smith CL, Taylor JR: Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 276 (1996) 1957-1963.
- Ferencik M, Ebringer L: Modulatory effects of selenium and zinc on the immune system. Folia Microbiol (Praha). 2003;48(3):417-26.
- Grippo JF, Holmgren A, Pratt WB: Proof that the endogenous, heat-stable glucocorticoid receptor-activating factor is thioredoxin. J Biol Chem. 1985 Jan 10;260(1):93-7.
- Hei YJ, Farahbakhshian S, Chen X, Battell ML, McNeill JH: Stimulation of MAP kinase and S6 kinase by vanadium and selenium in rat adipocytes. Mol Cell Biochem. 1998 Jan;178(1-2):367-75.
- Horvathova M, Jahnova E, Gazdik F: Effect of selenium supplementation in asthmatic subjects on the expression of endothelial cell adhesion molecules in culture. Biol Trace Elem Res. 1999 Jul;69(1):15-26.
- Hou JC: Inhibitory Effect of selenite and other antioxidants on complement-mediated tissue injury in patients with epidemic hemorrhagic fever. Biological Trace Element Research 56 (1997) 125-130.
- Kretz-Remy C, Arrigo AP. Selenium: a key element that controls NF-kappa B activation and I kappa B alpha half life. Biofactors. 2001;14(1-4):117-25.
- McKenzie RC, Arthur JR, Beckett GJ. Selenium and the regulation of cell signaling, growth, and survival: molecular and mechanistic aspects. Antioxid Redox Signal. 2002 Apr;4(2):339-51.
- Nève J: Methods in Determination of Selenium States. J. Trace Elem. Electrolytes Health Dis. 5 (1991) 1-17.
- Pillay TS, Makgoba MW: Enhancement of epidermal growth factor (EGF) and insulin-stimulated tyrosine phosphorylation of endogenous substrates by sodium selenate. FEBS Lett. 1992 Aug 10;308(1):38-42.
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001 Nov 8;345(19):1368-77.
- Rovensky J, Svik K, Stancikova M, Istok R, Ebringer L, Ferencik M: Treatment of experimental adjuvant arthritis with the combination of methotrexate and lyophilized Enterococcus faecium enriched with organic selenium. Folia Microbiol (Praha) 2002; 47(5):573-8.
- Sakr Y, Reinhart K, Bloos F, Marx G, Russwurm S, Bauer M, Brunkhorst F: Time course and relationship between plasma selenium concentrations, systemic inflammatory response, sepsis, and multiorgan failure. Br J Anaesth. 2007 Jun;98(6):775-84. Epub 2007 May 3.
- Stapleton SR, Garlock GL, Foellmi-Adams L, Kletzien RF: Selenium: potent stimulator of tyrosyl phosphorylation and activator of MAP kinase. Biochim Biophys Acta. 1997 Mar 1;1355(3):259-69.
- Thomas L (Hrsg.): Labor und Diagnose. 6. Auflage. TH-Books Verlagsgesellschaft mbH, Frankfurt/Main 2000.
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001 Nov 8;345(19):1359-67.
- Vitoux D, Forceville X, Gauzit R, Lahilaire P, Combes A, Chappuis P: Low plasma selenium in patients admitted in an intensive care unit is related to systemic inflammatory response syndrome and sepsis. Therapeutic Use of trace elements. Plenum Press, New York (1996) 127-131.
- Winnefeld K, Dawczynski H, Schirrmeister W, Adam G, Friedrich U, Hein S: Selenium in serum and whole blood in patients with surgical interventions. Biol Trace Elem Res 50 (1995) 149-155.
- Yang G, Yin S, Zhou R, Gu L, Yan B, Liu Y, Liu Y: Studies of safe maximal daily dietary Se-intake in a seleniferous area in China. Part II: Relation between Se-intake and the manifestation of clinical signs and certain biochemical alterations in blood and urine. J Trace Elem Electrolytes Health Dis 3 (1989) 123-130.
- Zhang F, Yu W, Hargrove JL, Greenspan P, Dean RG, Taylor EW, Hartle DK: Inhibition of TNF-alpha induced ICAM-1, VCAM-1 and E-selectin expression by selenium. Atherosclerosis. 2002 Apr;161(2):381-6.
- Zimmermann T, Albrecht S, Kühne H Vogelsang U, Grützmann R, Kopprasch S: Selensubstitution bei Sepsispatienten. Eine prospektiv randomisierte Studie. Med. Klin 92 (1997) (Suppl.III) 3-4.
Abbreviated Prescribing Information
selenase® 100 micrograms, solution for injection (50 micrograms/ml)
selenase® 500 micrograms, solution for injection (50 micrograms/ml)
- Active ingredient: sodium selenite pentahydrate.
- Composition: Each 2ml ampoule/10ml injection vial contains 100 micrograms/500 micrograms selenium as 333 micrograms/1.66mg sodium selenite penta- hydrate (Na2 SeO3 x 5H2O), corresponding to 50 micrograms/ml.
- Excipients: Sodium chloride, hydrochloric acid, Water for Injections.
- Indication: Proven selenium deficiency that cannot be offset from food sources.
- Posology and Administration: selenase® solution for injection is administered as an intramuscular or intravenous injection at a daily dose of 100 – 200 μg (1.27 – 2.53μmol) selenium. If necessary, this dose can be increased to 500 μg (6.33 μmol) for a typical adult. No dosage adjustment is required for paediatric, renal or hepatic impairment patients.
- Contraindications: Selenosis.
- Interactions: Ensure that the pH value does not fall below 7.0 and that the solution is not mixed with reducing substances (e.g. vitamin C).
- Pregnancy and Lactation: There are no data from the use of selenase in pregnant or lactating women.
- Undesirable Effects: None known to date when used as directed.
- Overdose: Counter measures include gastric lavage, forced diuresis, dialysis or administration of high doses of vitamin C.
- Pharmaceutical Precautions: Store below 25oC.
- Legal Category: POM.
- Presentation: Cartons containing 10 x 2ml ampoules / 10 x10ml glass vials for single use.
- MA Numbers: PL 20437/0003, PL 20437/0004.
- MA Holder: biosyn Arzneimittel GmbH, Schorndorfer Str 32, D-70734 Fellbach, Germany.
- Date of Preparation: November2004